News | December 16, 2008
Fuel cells on the fast track
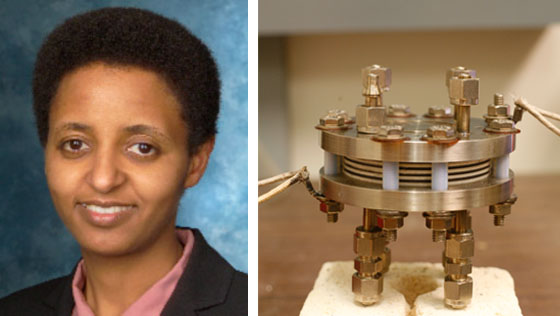
Left: Fuel cell pioneer Sossina Haile. Right: A stack of fuel cells created in Haile's lab. (Photo courtesy Superprotonic, Inc.)
The slow evolution of clean-energy solutions is about to kick into high gear, if Sossina M. Haile has anything to say about it. As a fuel cell researcher at the California Institute of Technology and a founding member of the company Superprotonic Inc., she hopes to make this “technology of the future” practical for today’s applications.

Haile’s research, which initially began several years ago with fuel cell researchers at JPL, has led to breakthroughs in more “consumer-ready” fuel cell technology. She’s developed fuel cell systems that strike a balance between power and manageability –- perfect, she says, for standalone residential generators. Her team has worked hard to reduce the amount of platinum needed for each system.
Haile's team has also taken on one of the biggest roadblocks to widespread fuel cell use -- their reliance on hydrogen as a primary fuel. Hydrogen requires lots of energy to extract and it’s difficult to store and distribute.

Haile's team has focused on developing fuel cells that can run on more traditional fuels, like ethanol or biomass, while also solving many of the problems of conventional hydrogen fuel cells.
Fuel cells that use carbon-based fuels still produce carbon emissions, but at a much lower rate than their internal-combustion counterparts. Because fuel cells extract energy from electrochemical reactions instead of burning their fuel, they are much more efficient and environmentally friendly. “It’s a unique middle ground,” explains Haile -- one she believes will speed the integration of these new technologies into the current energy infrastructure.
For Haile, the incentive to design practical, unconventional fuel cells is simple: “Science should be in the service of society.” She thinks that fuel cells that can use renewable energy resources like biomass will help end what she calls she calls “drawing from the bank” -- using fossil fuels as a source of energy.
“There’s scientific proof that CO2 concentrations have been rising for decades to levels not felt on the Earth in millenia,” Haile says. “We need to have a diverse approach to solving the problem before it’s too late.”
Written by Joshua Rodriguez/Global Climate Change
